Professor Oliver Bandmann
MD, PhD
School of Medicine and Population Health
Professor of Movement Disorders Neurology
Honorary Consultant Neurologist
Co-Director, Neuroscience Institute


+44 114 222 2237
Full contact details
School of Medicine and Population Health
Room B32
Sheffield Institute for Translational Neuroscience (SITraN)
385a Glossop Road
Sheffield
S10 2HQ
- Profile
-
- 2002-present: University of Sheffield/Royal Hallamshire Hospital, Sheffield, UK
- 1997-2002: Specialist Training in Neurology, Marburg, Germany;
- 1993-1997: Research Fellow, Institute of Neurology. Queen Square London. PhD on "Genetic Aspects of Parkinsonian Disorders" (Supervisors: Prof A.E. Harding, Prof C.D. Marsden, Prof N.W. Wood)
- 1992-1993: House officer, Dept Neurology, Klinikum Grosshadern, Munich (Head: Prof T. Brandt)
- 1991: Qualification at Ludwig Maximilian´s University Munich, Germany
- Research interests
-
My research focuses on movement disorders, in particular Parkinson´s Disease (PD) but also Huntington´s Disease, Wilson Disease and dystonia. I’m particularly interested in working towards disease-modifying therapy for PD which would slow down disease progression.
The main areas of research within my group are as follows:
1. Mitochondrial dysfunction and compound screen with identification of neuroprotective compounds as candidates for disease-modifying treatment in Parkinson’s Disease:
We were the first group worldwide to undertake detailed assessment of mitochondrial function and morphology in both parkin-mutant Parkinson’s Disease and LRRK2-G2019S mutant Parkinson’s Disease. We subsequently undertook the first compound screen in Parkinson’s Disease mutant patient tissue. 2000 drugs were assessed for their rescue effect on mitochondrial function. A clear mode of action (MOA) was identified for a group of compounds which includes the FDA-licensed drug ursodeoxycholic acid (UDCA).For interested members of the public: Please have a look at the official University of Sheffield press release for more information on this project. You may also be interested in listening to a BBC interview with Prof Bandmann.
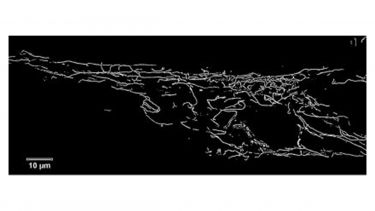
Fig1. The image shows a fibroblast from a patient with Parkinson’s Disease due to mutations in the parkin gene. The fibroblast has been stained to show the mitochondria in the cell. We see increased branching and interconnectedness of the mitochondrial network in the fibroblasts from patients with parkin mutations compared to controls; this change in morphology of the mitochondria correlates with changes in function.
2. Bench to bedside – early clinical trials in Parkinson’s disease
There is emerging evidence of a “Parkinson Epidemic” with a predicted global doubling in the number of people with Parkinson’s from 6 million to 12 million between 2015 and 2014 (PMID:30584159).
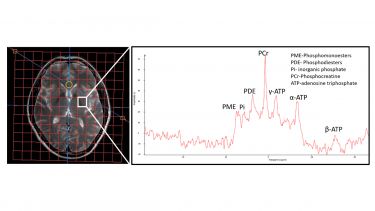
We have now taken UDCA as the top hit of our drug screen into an early clinical trial, called the UP study (short for: UDCA in Parkinson’s disease). The UP study will predominantly focus on investigating the safety and tolerability of UDCA in PD. However, we will also use novel techniques (in particular 31P-MR-Spectroscopy and sensor-based objective quantification of motor impairment) to gain some insight into the neuroprotective potential of UDCA.
MR Spectroscopy of a human brain with focussed analysis of the basal ganglia (the area most affected in Parkinson’s disease)
My group is also participating in two other multicentre neuroprotection trials, testing statins and antibodies against alpha-synuclein for their neuroprotective effect.
For interested members of the public: Please have a look at the official University of Sheffield Press release for more information on this project
https://www.sheffield.ac.uk/news/nr/liver-drug-trialled-in-parkinsons-patients-1.828628This link will take you to a webpage of the Cure Parkinson’s Trust - it provides additional information on UDCA for Parkinson’s:
https://www.cureparkinsons.org.uk/udca-and-pdYou may also find this video interesting during which we describe our journey from the bench at SITraN to the clinical trial carried out at the NIHR-funded Clinical Research facility at the Royal Hallamshire Hospital:
https://www.youtube.com/watch?v=izPA4-RBupw3. Zebrafish as a new vertebrate animal model for Parkinson’s Disease:
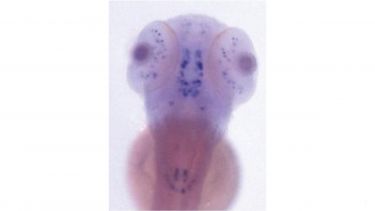
We were the first group worldwide to establish a zebrafish model of familial Parkinson’s Disease at Bateson Centre of the University of Sheffield (https://www.sheffield.ac.uk/bateson).
We subsequently demonstrated that Parkin-deficient zebrafish share key features with human parkin-mutant Parkinson’s Disease patients, namely mitochondrial dysfunction and loss of dopaminergic neurons. Most recently, we have identified up-regulation of TIGAR as novel mechanism leading to mitochondrial dysfunction and dopaminergic cell loss in PINK1 deficiency. Inhibition of TIGAR prevents loss of dopaminergic neurons by normalizing mitochondrial function. TIGAR is therefore a promising novel target for disease-modifying therapy in early onset Parkinson’s Disease. We are now also using zebrafish to study genetic risk factors for sporadic Parkinson’s and how they may interact with ageing.
For interested members of the public: Please have a look at the
official University of Sheffield press release for more information on this project.4. Mitochondrial biomarkers in Parkinson’s Disease:
We have just completed a detailed assessment of mitochondrial and lysosomal dysfunction in patients with sporadic Parkinson’s Disease. This will hopefully allow to eventually identify those patients with Parkinson’s Disease who are most likely to benefit from medication with mitochondrial rescue drugs.5. Huntington's Disease (HD)
We are recruiting patients for the Enroll-HD study at our multidisciplinary Huntington’s Disease clinic. As part of this, we have been frequently recruiting patients for HD drug trials and other HD studies.6. Wilson Disease (WD)
I have previously contributed to the development of the first validated clinical rating scale for the neurological impairment in Wilson Disease. More recently, we have conducted the first study on the genetic prevalence of Wilson Disease in the UK in collaboration with the Welcome Sanger Institute, Cambridge, UK. This study demonstrated a surprisingly high ATP7B carrier frequency which suggests that WD may be considerably more common than previously thought. In collaboration with the Sheffield Diagnostic Genetics Service, we also identified novel genetic mechanisms in WD patients.In close collaboration with the Wilson disease patient self-help group, we have helped to establish a UK-wide, interdisciplinary Wilson disease network. This network will hopefully allow us to develop national standards for the care of Wilson disease patients and facilitate future research projects.
- Publications
-
Journal articles
- Clinical Trial Highlights: Modulators of Mitochondrial Function. Journal of Parkinson's Disease, 13(6), 851-864.


- Outcome Measures for Disease-Modifying Trials in Parkinson’s Disease: Consensus Paper by the EJS ACT-PD Multi-Arm Multi-Stage Trial Initiative. Journal of Parkinson's Disease, 13(6), 1011-1033.


- The potential of innovative trial design for efficiently evaluating repurposed drugs. Nature Reviews Drug Discovery, 22(9), 681-682.


- 3α,7-Dihydroxy-14(13→12)abeo-5β,12α(H),13β(H)-cholan-24-oic Acids Display Neuroprotective Properties in Common Forms of Parkinson’s Disease. Biomolecules, 13(1).


- Increased fracture risk in Parkinson's disease – An exploration of mechanisms and consequences for fracture prediction with FRAX. Bone, 168, 116651-116651.


- Using 31-phosphorus magnetic resonance spectroscopy (31P-MRS) to identify Parkinson’s Disease subgroups with bioenergetic dysfunction. Journal of Neurology, Neurosurgery & Psychiatry, 93(9), e2.2-e2.2.


- Investigation and management of Wilson's disease: a practical guide from the British Association for the Study of the Liver. The Lancet Gastroenterology & Hepatology.


- Progress towards therapies for disease modification in Parkinson's disease. The Lancet Neurology, 20(7), 559-572.


- Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with Lewy bodies: Voxel-based morphometry and neuropsychological meta-analysis. Neuroscience & Biobehavioral Reviews.


- Cognitive correlates and baseline predictors of future development of visual hallucinations in dementia with Lewy bodies. Cortex.


- PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Scientific Reports, 11(1).


- Targeting mechanisms in cognitive training for neurodegenerative diseases. Neural Regeneration Research, 16(3), 500-500.


- Plasma neurofilament light as a biomarker of neurological involvement in Wilson's disease. Movement Disorders, 36(2), 503-508. View this article in WRRO


- Pathogenic Huntingtin Repeat Expansions in Patients with Frontotemporal Dementia and Amyotrophic Lateral Sclerosis. Neuron, 109(3), 448-460.e4.


- Ursodeoxycholic acid as a novel disease-modifying treatment for Parkinson’s disease: protocol for a two-centre, randomised, double-blind, placebo-controlled trial, The 'UP' study. BMJ Open, 10(8).


- Liver transplant for neurologic Wilson disease. Neurology, 94(21), 907-908.


- Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease. Progress in Neurobiology, 187, 101772-101772.


- Serum FGF-21, GDF-15, and blood mtDNA copy number are not biomarkers of Parkinson disease. Neurology: Clinical Practice, 10(1), 40-46.


- 136 Mitochondrial biomarkers in parkinson’s disease. Journal of Neurology Neurosurgery & Psychiatry, 90(12), e38.


- C9orf72 expansion within astrocytes reduces metabolic flexibility in amyotrophic lateral sclerosis. Brain, 1-20. View this article in WRRO


- Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson's disease. Biology Open, 8(10). View this article in WRRO


- The genetic and clinico‐pathological profile of early‐onset progressive supranuclear palsy. Movement Disorders, 34(9), 1307-1314.


- Ablation of the pro-inflammatory master regulator miR-155 does not mitigate neuroinflammation or neurodegeneration in a vertebrate model of Gaucher's disease. Neurobiology of Disease, 127, 563-569.


- Meaningful and Measurable Health Domains in Huntington’s Disease: Large-Scale Validation of the Huntington’s Disease Health-Related Quality of Life Questionnaire Across Severity Stages. Value in Health, 22(6), 712-720.


- Assessment of the Performance of a Modified Motor Scale as Applied to Juvenile Onset Huntington’s Disease. Journal of Huntington's Disease, 8(2), 181-193.


- Identification of symbol digit modality test score extremes in Huntington's disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(3), 232-245.


- Reduced habit-driven errors in Parkinson’s Disease. Nature Scientific Reports, 9. View this article in WRRO


- TIGAR inclusion pathology is specific for Lewy body diseases. Brain Research, 1706, 218-223. View this article in WRRO


- Clinical and genetic characteristics of late-onset Huntington's disease. Parkinsonism & Related Disorders, 61, 101-105.


- The effect of hyperglycemia on neurovascular coupling and cerebrovascular patterning in zebrafish. Journal of Cerebral Blood Flow and Metabolism. View this article in WRRO


- Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with either Sporadic or Familial Alzheimer's Disease. Journal of Molecular Biology, 430(21), 3942-3953. View this article in WRRO


- The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson’s Disease. Cell Reports, 23(10), 2976-2988.


- Parkinson’s Disease in Sub-Saharan Africa: A Review of Epidemiology, Genetics and Access to Care. Journal of Movement Disorders, 11(2), 53-64. View this article in WRRO


- Computer-aided diagnosis for (123I)FP-CIT imaging: impact on clinical reporting.. EJNMMI Research, 8(1). View this article in WRRO


- Porphyria: often discussed but too often missed. Practical Neurology, 18(5), 352-358.


- Translational approaches to restoring mitochondrial function in Parkinson's disease. FEBS Letters, 592(5), 776-792. View this article in WRRO


- Animal models of Wilson disease. Journal of Neurochemistry, 146(4), 356-373.


- The subresolution DaTSCAN phantom. Nuclear Medicine Communications, 39(3), 268-275. View this article in WRRO


- Reduced Cancer Incidence in Huntington's Disease: Analysis in the Registry Study. Journal of Huntington's Disease, 7(3), 209-222.


- PO076 Disease stratification in sporadic parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 88(Suppl 1), A31.4-A32.


- Cognitive decline in Huntington's disease expansion gene carriers. Cortex, 95, 51-62.


- Identification of genetic variants associated with Huntington's disease progression: a genome-wide association study. The Lancet Neurology, 16(9), 701-711.


- Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behaviour disorder. Sleep, 40(8). View this article in WRRO


- Structural and Functional Neuroimaging of Visual Hallucinations in Lewy Body Disease: A Systematic Literature Review.. Brain Sciences, 7(7). View this article in WRRO


- [P4-034]: MITOCHONDRIAL ABNORMALITIES ARE FOUND IN FIBROBLASTS FROM SPORADIC ALZHEIMER's DISEASE PATIENTS: RECOVERY WITH URSODOXYCHOLIC ACID TREATMENT. Alzheimer's & Dementia, 13(7S_Part_26), P1269-P1269.


- Epidemiology and introduction to the clinical presentation of Wilson disease.. Handb Clin Neurol, 142, 7-17.


- Inhibition of the mitochondrial calcium uniporter (MCU) rescues dopaminergic neurons in pink1-/- zebrafish. European Journal of Neuroscience, 45(4), 528-535. View this article in WRRO


- Genetics of multiple system atrophy: Back to square one?. Neurology.


- Rescue of mitochondrial function in parkin-mutant Fibroblasts using drug loaded PMPC-PDPA polymersomes and tubular polymersomes. Neuroscience Letters, 630, 23-29. View this article in WRRO


- Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death. Human Molecular Genetics, 24(23), 6640-6652. View this article in WRRO


- UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2G2019S carriers and in vivo. Neurology, 85(10), 846-852.


- Zebrafish (Danio rerio) as a model for Glucocerebrosidase 1 (GBA1) deficiency (S7.004). Neurology, 84(14_supplement).


- Wilson's disease and other neurological copper disorders. The Lancet Neurology, 14(1), 103-113.


- PH-sensitive tubular polymersomes: Formation and applications in cellular delivery. ACS Nano, 8(5), 4650-4661.


- TIGAR Inhibition Rescues Dopaminergic Neurons in Common Forms of Early Onset Parkinson's' Disease (I5-2.003). Neurology, 82(10_supplement).


- TIGAR Inhibition Rescues Dopaminergic Neurons in Common Forms of Early Onset Parkinson's' Disease (S7.002). Neurology, 82(10_supplement).


- The documentation of consent and disclosure of neurogenetic testing outside clinical genetics.. Neurogenetics, 15(1), 19-21.


- TIGARB CAUSES MITOCHONDRIAL DYSFUNCTION AND NEURONAL LOSS IN PINK1 DEFICIENCY.. J Neurol Neurosurg Psychiatry, 84(11), e2.


- Suicidal ideation in a European Huntington's disease population. Journal of Affective Disorders, 151(1), 248-258.


- Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson's disease.. Brain, 136(Pt 10), 3038-3050.


- TigarB causes mitochondrial dysfunction and neuronal loss in PINK1 deficiency.. Ann Neurol, 74(6), 837-847. View this article in WRRO


- C9ORF72 expansions, parkinsonism, and Parkinson disease: a clinicopathologic study.. Neurology, 81(9), 808-811.


- The V471A Polymorphism in Autophagy-Related Gene ATG7 Modifies Age at Onset Specifically in Italian Huntington Disease Patients. PLoS ONE, 8(7). View this article in WRRO


- Transglutaminase 6 antibodies in the diagnosis of gluten ataxia.. Neurology, 80(19), 1740-1745.


- Delayed toxic-hypoxic encephalopathy.. Pract Neurol, 13(2), 114-119.


- β-Defensin Genomic Copy Number Does Not Influence the Age of Onset in Huntington's Disease.. J Huntingtons Dis, 2(1), 107-124.


- A genetic study of Wilson's disease in the United Kingdom.. Brain, 136(Pt 5), 1476-1487.


- Biomarkers for PD: How can we approach complexity?. Neurology, 80(7), 608-609.


- β-Defensin Genomic Copy Number Does Not Influence the Age of Onset in Huntington's Disease. Journal of Huntington's Disease, 2(1), 107-124.


- Neuronal dark matter: the emerging role of microRNAs in neurodegeneration.. Front Cell Neurosci, 7, 178. View this article in WRRO


- Heterozygous mutations in the FGF8, SHH and nodal/transforming growth factor beta pathways do not confer increased dopaminergic neuron vulnerability--a zebrafish study.. Neurosci Lett, 532, 55-58.


- CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology, 78(10), 690-695.


- Parkinson disease, cancer, and LRRK2: causation or association?. Neurology, 78(11), 772-773.


- The Prevalence of Juvenile Huntington's Disease: A Review of the Literature and Meta-Analysis.. PLoS Curr, 4, e4f8606b742ef3.


- Discrepancies in reporting the CAG repeat lengths for Huntington's disease. European Journal of Human Genetics, 20(1), 20-26.


- Prolonged generalized dystonia after chronic cerebellar application of kainic acid. Brain Research.


- Prolonged generalized dystonia after chronic cerebellar application of kainic acid. Brain Research, 1464, 82-88.


- Observing Huntington's disease: the European Huntington's Disease Network's REGISTRY. Journal of Neurology, Neurosurgery & Psychiatry, 82(12), 1409-1412.


- The influence of the zebrafish genetic background on Parkinson's disease-related aspects.. Zebrafish, 8(3), 103-108.


- Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2.. Neurology, 75(22), 2017-2020.


- Genetic zebrafish models of neurodegenerative diseases.. Neurobiol Dis, 40(1), 58-65.


- Parkinson's disease: insights from pathways.. Hum Mol Genet, 19(R1), R21-R27.


- Corpus Callosum Morphology and Microstructure Assessed Using Structural MR Imaging and Diffusion Tensor Imaging: Initial Findings in Adults with Neurofibromatosis Type 1. AM J NEURORADIOL, 31(5), 856-861.


- Normal and mutant HTT interact to affect clinical severity and progression in Huntington disease. Neurology, 73(16), 1280-1285.


- Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss.. Nat Neurosci, 12(9), 1129-1135. View this article in WRRO


- Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio).. Brain, 132(Pt 6), 1613-1623.


- 7. Mitochondrial function and morphology are impaired in parkin mutant fibroblasts. Mitochondrion, 9(1), 63-63.


- Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts.. Ann Neurol, 64(5), 555-565.


- Zebrafish as a new animal model for movement disorders.. J Neurochem, 106(5), 1991-1997.


- Yet another spinocerebellar ataxia: the saga continues.. Neurology, 71(8), 542-543.


- Immunophenotyping in Tourette syndrome--a pilot study.. Eur J Neurol, 15(7), 749-753.


- Complicated autosomal recessive hereditary spastic paraplegia: a complex picture is emerging.. Neurology, 70(16 Pt 2), 1375-1376.


- Evaluation of the Unified Wilson's Disease Rating Scale (UWDRS) in German patients with treated Wilson's disease.. Mov Disord, 23(1), 54-62.


- p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease.. J Neurochem, 100(6), 1626-1635.


- Unified Wilson's Disease Rating Scale (UWDRS) - a proposal for the neurological scoring of Wilson's disease patients. PARKINSONISM RELAT D, 13, S80-S80.


- Unified Wilson's Disease Rating Scale - a proposal for the neurological scoring of Wilson's disease patients.. Neurol Neurochir Pol, 41(1), 1-12.


- CNS involvement in hereditary neuropathy with pressure palsies (HNPP).. Neurology, 67(12), 2250-2252.


- Prevalence of the H1069Q mutation in ATP7B in discordant pairs with early-onset Parkinson's disease.. Mov Disord, 21(10), 1789-1790.


- Brain-derived neurotrophic factor: a genetic risk factor for obsessive-compulsive disorder and Tourette syndrome?. Mov Disord, 21(6), 881-883.


- Lack of association with TorsinA haplotype in German patients with sporadic dystonia.. Neurology, 66(6), 951-952.


- The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group.. Brain, 128(Pt 8), 1855-1860.


- Neurodegenerative disorders: Parkinson's disease and Huntington's disease.. J Neurol Neurosurg Psychiatry, 76(8), 1058-1063.


- Epsilon-sarcoglycan is not involved in sporadic Gilles de la Tourette syndrome.. Neurogenetics, 6(1), 55-56.


- Dopamine receptor gene polymorphisms in Parkinson's disease patients reporting "sleep attacks".. Mov Disord, 19(11), 1279-1284.


- Advances in dystonia. EUR J NEUROL, 11, 1-1.


- Slow N-acetyltransferase 2 status leads to enhanced intrastriatal dopamine depletion in 6-hydroxydopamine-lesioned rats.. Exp Neurol, 187(1), 199-202.


- Lack of association between the interleukin-1 alpha (-889) polymorphism and early-onset Parkinson's disease.. Neurosci Lett, 359(3), 195-197.


- DJ-1: the second gene for early onset Parkinson disease.. Neurology, 62(3), 357-358.


- Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients.. Mov Disord, 19(2), 192-196.


- Candidate gene studies in focal dystonia.. Neurology, 61(8), 1097-1101.


- HLA-DRB genotyping in Gilles de la Tourette patients and their parents.. Am J Med Genet B Neuropsychiatr Genet, 119B(1), 60-64.


- The phenylalanine loading test in the differential diagnosis of dystonia.. Neurology, 60(4), 700-702.


- The promoter region of the Menkes gene ATP7A is not altered in focal or generalized dystonia.. Ann Neurol, 53(2), 278-279.


- Myoclonic encephalopathy caused by chronic bismuth abuse.. Epileptic Disord, 4(4), 229-233.


- Copper genes are not implicated in the pathogenesis of focal dystonia.. Neurology, 59(5), 782-783.


- Spectrum of mutations in the gene for epsilon-sarcoglycan (SGCE) in myoclonus-dystonia syndrome (MDS, DYT11). NEUROLOGY, 58(7), A17-A17.


- Steele-Richardson-Olszewski-syndrome: the relation of dopamine D2 receptor binding and subcortical lesions in MRI.. J Neural Transm (Vienna), 109(4), 503-512.


- Dopa-responsive dystonia -- the story so far.. Neuropediatrics, 33(1), 1-5.


- Detailed genotyping demonstrates association between the slow acetylator genotype for N-acetyltransferase 2 (NAT2) and familial Parkinson's disease.. Mov Disord, 15(1), 30-35.


- Different postural reaction patterns for expected and unexpected perturbations in patients with idiopathic Parkinson's disease and other parkinsonian syndromes.. Eur J Neurol, 6(5), 549-554.


- The tau gene A0 polymorphism in progressive supranuclear palsy and related neurodegenerative diseases.. J Neurol Neurosurg Psychiatry, 66(5), 665-667.


- [The therapy of the parkinsonian syndrome].. Dtsch Med Wochenschr, 124(8), 219-222.


- GTP cyclohydrolase deficiency; intrafamilial variation in clinical phenotype, including levodopa responsiveness.. J Neurol Neurosurg Psychiatry, 66(1), 86-89.


- Multiple system atrophy.. J Neural Transm Suppl, 56, 155-164.


- Toxins, genetics, and Parkinson's disease: the role of N-acetyltransferase 2.. Adv Neurol, 80, 199-204.


- Dopa-responsive dystonia: a clinical and molecular genetic study.. Ann Neurol, 44(4), 649-656.


- The alpha-synuclein Ala53Thr mutation is not a common cause of familial Parkinson's disease: a study of 230 European cases. European Consortium on Genetic Susceptibility in Parkinson's Disease.. Ann Neurol, 44(2), 270-273.


- Mental disorders in movement disorders. CURR OPIN PSYCHIATR, 11(3), 315-319.


- Genetic aspects of Parkinson's disease.. Mov Disord, 13(2), 203-211.


- Acetylator genotype and Parkinson's disease - Reply. LANCET, 351(9096), 142-142.


- Acetylator genotype and Parkinson's disease (multiple letters) [13]. Lancet, 351(9096), 141-142.


- Atypical presentations of dopa-responsive dystonia.. Adv Neurol, 78, 283-290.


- Multiple-system atrophy is genetically distinct from identified inherited causes of spinocerebellar degeneration.. Neurology, 49(6), 1598-1604.


- Association of slow acetylator genotype for N-acetyltransferase 2 with familial Parkinson's disease.. Lancet, 350(9085), 1136-1139.


- GTP cyclohydrolase I mutations in patients with dystonia responsive to anticholinergic drugs.. J Neurol Neurosurg Psychiatry, 63(3), 304-308.


- Mitochondrial DNA polymorphisms in pathologically proven Parkinson's disease.. J Neurol, 244(4), 262-265.


- The GTP-cyclohydrolase I gene in atypical parkinsonian patients: a clinico-genetic study.. J Neurol Sci, 141(1-2), 27-32.


- The human homologue of the weaver mouse gene in familial and sporadic Parkinson's disease.. Neuroscience, 72(4), 877-879.


- Debrisoquine hydroxylase polymorphism in Leber's hereditary optic neuropathy.. J Neurol Neurosurg Psychiatry, 60(5), 588.


- Dopa-responsive dystonia in British patients: new mutations of the GTP-cyclohydrolase I gene and evidence for genetic heterogeneity.. Hum Mol Genet, 5(3), 403-406.


- Signal changes on MRI and increases in reactive microgliosis, astrogliosis, and iron in the putamen of two patients with multiple system atrophy.. J Neurol Neurosurg Psychiatry, 60(1), 98-101.


- Peripheral markers in Parkinson's disease. An overview.. Adv Neurol, 69, 283-291.


- Arg296 to Cys296 polymorphism in exon 6 of cytochrome P-450-2D6 (CYP2D6) is not associated with multiple system atrophy.. J Neurol Neurosurg Psychiatry, 59(5), 557.


- Sequence of the superoxide dismutase 1 (SOD 1) gene in familial Parkinson's disease.. J Neurol Neurosurg Psychiatry, 59(1), 90-91.


- CYP2D6-debrisoquine hydroxylase gene polymorphism in multiple system atrophy.. Mov Disord, 10(3), 277-278.


- Epidemiological, genetic, pharmacological, kinesiological, nuclear medical (IBZM-SPECT), standard and functional MRI studies on Parkinson's disease and related disorders and economic evaluation of Parkinson's disease therapy--clinical projects in the BMFT-research program Munich: "Parkinson's disease and other basal ganglia disorders".. J Neural Transm Suppl, 46, 325-337.


- Apo E genotypes in multiple sclerosis, Parkinson's disease, schwannomas and late-onset Alzheimer's disease.. Mol Cell Probes, 8(6), 519-525.


- A contrast enhanced lesion of the III nerve on MR of a patient with ophthalmoplegic migraine as evidence for a Tolosa-Hunt syndrome.. Headache, 33(8), 446-448.


- 3H-spiperone binding to lymphocytes fails in the differential diagnosis of de novo Parkinson syndromes.. J Neural Transm Park Dis Dement Sect, 5(2), 107-116.


- Parkinson’s families project: a UK-wide study of early onset and familial Parkinson’s disease. npj Parkinson's Disease, 10(1).


- Polygenic Risk Scores Validated in Patient‐Derived Cells Stratify for Mitochondrial Subtypes of Parkinson's Disease. Annals of Neurology.


- Multimodal assessment of mitochondrial function in Parkinson's disease. Brain.


- The master energy homeostasis regulator PGC-1α exhibits an mRNA nuclear export function. Nature Communications, 14(1).


- A Double‐Blind, Randomized, Placebo‐Controlled Trial of Ursodeoxycholic Acid (
UDCA) in Parkinson's Disease. Movement Disorders.

- Unexpected phenotypic and molecular changes of combined glucocerebrosidase and acid sphingomyelinase deficiency. Disease Models & Mechanisms.


- Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain.


- Neuroimaging Correlates of Cognitive Deficits in Wilson's Disease. Movement Disorders.


- GCH1 deficiency activates brain innate immune response and impairs tyrosine hydroxylase homeostasis. The Journal of Neuroscience.


- Wilson’s disease: update on pathogenesis, biomarkers and treatments. Journal of Neurology, Neurosurgery & Psychiatry.


- Neuroimaging correlates of brain injury in Wilson’s disease: a multimodal, whole-brain MRI study. Brain.


- Neurological letter from Bangladesh. Practical Neurology.


- NMDA receptor gene variations as modifiers in Huntington disease: a replication study. PLoS Currents, 3, RRN1247-RRN1247.


- Huntington disease. Neurology, 87(3), 247-248.


- The common PARK8 mutation LRRK2 G2019S is not a risk factor for breast cancer in the absence of Parkinson's disease.. Journal of Neurology.


Chapters
- Neurodegenerative Disorders: Parkinson's Disease and Huntington's Disease, Neuroscience for Neurologists (pp. 181-200). PUBLISHED BY IMPERIAL COLLEGE PRESS AND DISTRIBUTED BY WORLD SCIENTIFIC PUBLISHING CO.


Conference proceedings papers
- I026 Screening for mitochondrial therapeutics in Huntington’s disease using patient-derived cells. I: Experimental therapeutics – preclinical (pp A152.1-A152)


- Repurposing Anti-Gout Medications for the Treatment of Parkinson's disease (PD). MOVEMENT DISORDERS, Vol. 39 (pp S402-S402)


- Treatment Selection in Multi-Arm Multi-Stage Clinical Trials in Parkinson Disease: The Search for the Ideal Neuroprotective Drug. MOVEMENT DISORDERS, Vol. 37 (pp S329-S329)


- Multimodal mechanistic disease stratification in sporadic Parkinson's disease. MOVEMENT DISORDERS, Vol. 37 (pp S113-S113)


- Developing 31-phosphorus magnetic resonance spectroscopy (31P-MRS) as an imaging biomarker to identify mitochondrial dysfunction in Parkinson's disease. MOVEMENT DISORDERS, Vol. 36 (pp S370-S371)


- (31)Phosphorus Magnetic Resonance Spectroscopy as a Tool to Identify Mitochondrial Dysfunction in Parkinson's Disease In-Vivo. ANNALS OF NEUROLOGY, Vol. 90 (pp S152-S153)


- The UP study - Ursodeoxycholic acid (UDCA) as neuroprotective treatment for Parkinson's disease. MOVEMENT DISORDERS, Vol. 35 (pp S413-S414)


- Disease-modifying treatment in Parkinson's disease - opportunities and obstacles. FEBS OPEN BIO, Vol. 9 (pp 40-40)


- Adult neurogenesis is impaired in pink1-/- zebrafish (Danio rerio). MOVEMENT DISORDERS, Vol. 33 (pp S605-S605)


- Acid sphingomyelinase deficiency rescues mitochondrial dysfunction in gba-/- zebrafish (Danio rerio). MOVEMENT DISORDERS, Vol. 33 (pp S615-S615)


- Impact of Computer Aided Diagnosis (CAD) on DaTSCAN reporting: a pilot study. EUROPEAN JOURNAL OF NUCLEAR MEDICINE AND MOLECULAR IMAGING, Vol. 44 (pp S611-S611)


- The role of TIGAR in Parkinson's disease. NEUROPATHOLOGY AND APPLIED NEUROBIOLOGY, Vol. 42 (pp 34-35)


- MPP+ IN A ZEBRAFISH MODEL OF GLUCOCEREBROSIDASE 1 DEFICIENCY. Journal of Neurology, Neurosurgery & Psychiatry, Vol. 86(11) (pp e4.93-e4)


- ZEBRAFISH AS A MODEL OF GLUCOCEREBROSIDASE 1 (GBA1) DEFICIENCY. Journal of Neurology, Neurosurgery & Psychiatry, Vol. 86(11) (pp e4.46-e4)


- Deep learning for automatic cell detection in wide-field microscopy zebrafish images. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), 16 April 2015 - 19 April 2015.


- Three-Dimensional Deconvolution of Wide Field Microscopy with Sparse Priors: Application to Zebrafish Imagery. 2014 22nd International Conference on Pattern Recognition, 24 August 2014 - 28 August 2014.


- TIGAR inactivation rescues dopaminergic neurons in parkin deficiency. MOVEMENT DISORDERS, Vol. 29(14) (pp 1839-1840)


- TIGAR inactivation rescues dopaminergic neurons in parkin deficiency. MOVEMENT DISORDERS, Vol. 29 (pp S3-S3)


- Neurodegeneration caused by intronic expansions of C9ORF72 is a clinically heterogeneous but pathologically distinct disease. LANCET, Vol. 381 (pp 32-32)


- A NIGHT TO REMEMBER: ALL WHITE THEN, ALL RIGHT NOW?. JOURNAL OF NEUROLOGY NEUROSURGERY AND PSYCHIATRY, Vol. 83


- Mitochondrial Impairment in Manifesting LRRK2-G2019S Carriers. NEUROLOGY, Vol. 74(9) (pp A255-A255)


- Bone marrow transplantation in adult cerebral X-linked adrenoleukodystrophy: an update. BONE MARROW TRANSPLANTATION, Vol. 43 (pp S182-S183)


- Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio). MOVEMENT DISORDERS, Vol. 24 (pp S135-S135)


- Abnormal mitochondrial function and morphology in fibroblasts of patients with early onset Parkinson's disease and two parkin mutations. NEUROLOGY, Vol. 70(11) (pp A485-A485)


- Mitochondrial function and morphology in parkin mutant fibroblasts. MOVEMENT DISORDERS, Vol. 23(1) (pp S49-S49)


- P53 dependent neuronal cell death in a DJ-1 deficient zebrafish model of Parkinson disease. JOURNAL OF NEUROLOGY NEUROSURGERY AND PSYCHIATRY, Vol. 78(9) (pp 1024-1024)


- Proximal and central myelin damage of cranial nerves in hereditary neuropathy with liability to pressure palsies. NEUROLOGY, Vol. 64(6) (pp A376-A376)


- Immunocytological analysis of B-, T-, and natural killer cell subsets in Tourette syndrome. MOVEMENT DISORDERS, Vol. 19 (pp S439-S439)


- The Va166Met polymorphism of the brain derived neurotrophic factor (BDNF): A shared genetic risk factor for obsessive-compulsive behaviour and Gilles de la Tourette syndrome?. MOVEMENT DISORDERS, Vol. 19 (pp S354-S354)


- Prevalence of UCHL1, DJ1 and NR4A2 gene mutations in young-onset Parkinson's disease (YOPD) patients. MOVEMENT DISORDERS, Vol. 19 (pp S358-S358)


- The role of the epsilon-sarcoglycan gene (SGCE) in Gilles de la Tourette patients. MOVEMENT DISORDERS, Vol. 19 (pp S363-S363)


- Evaluation of the epsilon-sarcoglyan (SGCE) promoter region in myoclonus-dystonia (M-D). MOVEMENT DISORDERS, Vol. 19 (pp S96-S97)


- Gene expression studies in a novel rat dystonia model. MOVEMENT DISORDERS, Vol. 19 (pp S98-S98)


- The role of CNS dopaminergic, serotonergic and hypocretin (Orexin) systems in restless legs syndrome. SLEEP, Vol. 26 (pp A325-A326)


- Tourette syndrome and attention deficit hyperactivity disorder: Are there shared genetic susceptibility factors?. MOVEMENT DISORDERS, Vol. 17 (pp S342-S342)


- Dopamine receptor and hypocretin gene polymorphisms in Parkinson's disease (PD) patients reporting "sleep attacks". MOVEMENT DISORDERS, Vol. 17 (pp S138-S138)


- Candidate gene research in focal dystonia excludes involvement of ATP7A, ATP7B, ATOX, HLA-DR and MTHFR. MOVEMENT DISORDERS, Vol. 17 (pp S278-S279)


Preprints
- Integrating Natural Language Processing to Assess Effect of Loneliness and Social isolation on Cognitive Decline in Dementia: A Retrospective Cohort Analysis, Cold Spring Harbor Laboratory.


- The master energy homeostasis regulator PGC-1α couples transcriptional co-activation and mRNA nuclear export, Cold Spring Harbor Laboratory.


- Acid Sphingomyelinase Deficiency Normalizes Neuronal Function in GCase Deficiency - Unexpected Biological Rescue Effect of Combined Genetic Risk Factors for Parkinson’s Disease, Research Square Platform LLC.


- Unexpected opposing biological effect of genetic risk factors for Parkinson’s disease, Cold Spring Harbor Laboratory.


- Reduced habit-driven errors in Parkinson’s Disease, Center for Open Science.


- Clinical Trial Highlights: Modulators of Mitochondrial Function. Journal of Parkinson's Disease, 13(6), 851-864.
- Research group
-
- Clinical Fellows: Dr Tom Payne, Dr Emily Reed
- UP study Trial Manager: Sarah Moll (co-funded by Sheffield Neuroscience BRC)
- Post-doctoral scientists: Dr Lisa Watson (ne Trollope), Dr Deepak Ailandi
- PhD students as primary supervisor: Mohammed Karami, Hannah Larbalestier, Emma White
Co-supervision with Dr Heather Mortiboys:
- Postdoctoral scientist: Dr Helen Rowland
- PhD students: Chris Hastings, Ruby McDonald, Rachel Hughes
- Technician: Sarah Roscoe
- Grants
-
The UP Study is predominantly funded by the JP Moulton Foundation, but also supported by Cure Parkinson’s UK and the Sheffield Neuroscience BRC.
My research is also supported by the Medical Research Council (MRC), the Michael J Fox Foundation (MJFF) and the University of Sheffield. In the past, I’ve also had substantial funding from Parkinson’s UK.
- Teaching interests
-
I was the Dept Neuroscience Undergraduate Teaching Lead/Director for Teaching and Learning for 15 years (2002-2017). I was also the University of Sheffield BMedSci Programme Director for 5 years (2013-2018).
I twice won the “Consultant Teacher of the Year" award of the Sheffield Medical Student Society MedSoc.
I continue to contribute to all undergraduate phases of our MBChB course and also contribute to SITRaN-based MSc courses.
I am now the academic training lead for the STH Neuroscience Directorate and also the Training Lead for our NIHR-funded Sheffield Neuroscience BRC.
- Professional activities and memberships
-
- Member of the Editorial Board of the clinical Neuroscience Journals NEUROLOGY and Parkinsonism& Related Disorders
- Member of the MRC-DPFS Panel
- Deputy Chair of Association of British Neurologists (ABN) Research Committee
- Chair of the ABN Movement Disorders Advisory Group
- Movement Disorders Theme Lead of STH Academic Neuroscience Directorate and the NIHR-funded Sheffield Neuroscience BRC
Awards and esteem factors
- 1984-1991: Bavarian Scholarship for highly gifted students
- 1986-1991: German Scholarship for highly gifted students ("Studienstiftung des Deutschen Volkes")
- 1993-1997: Training Fellowship of Deutsche Forschungsgemeinschaft (DFG)
- 1996: Presidents Prize of Royal Society of Medicine
- 1997: Queen Square Prize of Institute of Neurology
- 2000: Oppenheim Prize of German Dystonia Society
- 2006: "Consultant Teacher of the Year" Prize of "Med Soc" (organization of medical students at UoS Medical School)