- University of Sheffield researchers have developed a new way of testing biomaterials that could help to avoid a repeat of the vaginal mesh scandal
- New testing method could form the basis of an early-warning system to flag biomaterials that are not suitable for clinical use inside the human body
- Polypropylene mesh surgery has left thousands of women with life-changing complications following its use to treat pelvic organ prolapse and stress urinary incontinence
- Study has revealed new insights into why polypropylene mesh was inappropriate for clinical use within the pelvic floor
A new way of testing biomaterials that could help to avoid a repeat of the vaginal mesh scandal, has been developed by researchers at the University of Sheffield.
In a study led by Dr Nicholas Farr from the University’s Department of Materials Science and Engineering, researchers have developed an oxidative stress test that can form the basis of an early-warning system to identify biomaterials that are not suitable for clinical use inside the human body.
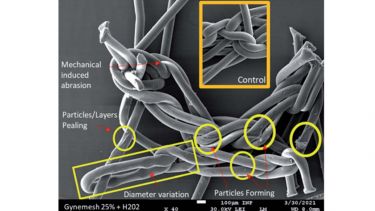
Published in the journal RSC Advances, the new method can better simulate deployment environments - such as those inside the human body - and identify cracks and surface degradation in biomaterials far better than the methods currently used.
The test subjects biomaterials to oxidation and mechanical stresses, both conditions they would experience inside a patient. This can give the makers behind biomaterials a better understanding of how their materials will perform over time in clinical use and any issues that are likely to occur should they be used as a medical treatment.
The researchers developed their procedure by evaluating a type of surgical mesh made of polypropylene - also known as PP mesh.
PP mesh has been used to treat pelvic organ prolapse and stress urinary incontinence - a condition that affects 50 per cent of postmenopausal women worldwide. However, the use of PP mesh has caused life-changing complications in thousands of women and led to medical negligence lawsuits.
Whilst manufacturers assert that the PP material is inert and non-degradable, there is an increasing body of evidence that reveals PP meshes are subject to oxidative damage. This is further supported by explanted material from patients suffering with clinical complications showing evidence of fibre cracking and oxidation.
The new testing method developed by the Sheffield researchers could form the basis of an early-warning system against these types of failures as it is capable of detecting inappropriate material at the nanoscale.
Dr Nicholas Farr, EPSRC Doctoral Research Fellow at the University of Sheffield, said: “The process starts with subjecting the material to conditions which mimic the natural processes of oxidation and mechanical stress experienced within the human body. This is followed by analysis using novel material characterisation techniques to evaluate the nanoscale surface of the material. It is envisioned that the process outlined has the potential to form the basis of an "early warning" analysis system which possesses the ability to identify materials which are not suitable for clinical deployment within the human body. My hope is that this research can not only aid the development of new implantable materials but also help to update the regulatory standards which govern medical device production.”
Using this new technique, the Sheffield researchers have been able to further investigate why the PP mesh was not suitable for use in the pelvic floor.
The researchers found that the stress loaded onto the PP mesh while inside the body is likely to have caused polymer oxidation and chemical reactions in the materials - this oxidation and these reactions will have likely changed the material’s molecular structure, causing the material to crack and the release of etched oxidised insoluble particles initiating an inflammatory response in the body.
Emeritus Professor Sheila MacNeil, who has worked with NHS Consultant Professor Chris Chapple to develop alternative safer materials for use in the pelvic floor, added: “The polypropylene material used to provide support in the pelvic floor is very strong but has produced severe complications in many women. In this study we looked to see how the material performed under simulated stress conditions.
“We stretched the material repeatedly for three days, subjected it to mild oxidative stress and then we used the very sensitive test to reveal tiny fissures and cracks in the surface of the material. Our extensive study has shown this material does not cope well under pressure.”
The study, A novel characterisation approach to reveal the mechano–chemical effects of oxidation and dynamic distension on polypropylene surgical mesh, is published in RSC Advances. Access the paper.
Contact
For further information please contact: