Dr Daniel Humphreys
School of Biosciences
Senior Research Fellow
+44 114 222 4632
Full contact details
School of Biosciences
Florey Building
Western Bank
Sheffield
S10 2TN
- Profile
-
My research focusses on the host cell biology underlying infectious diseases with the aim of discovering new ways to combat globally important bacterial pathogens. I am a UKRI Future Leaders Research Fellow studying DNA damage responses and senescence in typhoid fever and its transmission by chronic bacterial carriage.
I obtained my PhD and gained postdoctoral training at the University of Cambridge before being appointed as a lecturer in 2016 at the School of Biosciences (formerly the Department of Biomedical Science), University of Sheffield, where I received an MRC New Investigator Research Grant. Before that I studied Microbiology at the University of Liverpool (BSc) and Biological Sciences at the University of Manchester (Masters).
- 2022 - present: Senior Lecturer, School of Biosciences, University of Sheffield
- 2019 - present: UKRI Future Leaders Research Fellow
- 2016 - 2022: Lecturer, School of Biosciences, University of Sheffield
- 2014 - 2016: Research Fellow, Department of Pathology, University of Cambridge
- 2008 - 2013: Postdoctoral Research Associate, University of Cambridge
- 2003 - 2007: PhD in Cellular Microbiology, Department of Pathology, University of Cambridge
- 2002 - 2003: Masters of Biological Research, University of Manchester
- 1999 - 2002: BSc in Microbiology, University of Liverpool
- Research interests
-
Typhoid fever and its transmission by chronic bacterial carriage
Typhoid fever is a major disease in low- and middle-income countries resulting in ~11 million cases and ~128,000 deaths each year. Typhoid is caused by the human-restricted pathogen Salmonella Typhi that has evolved antibiotic-resistance with multi- and extremely-resistant strains causing current epidemics. Please have a look at our typhoid animation for more information: https://www.youtube.com/watch?v=SrCjvP05mlQ
S.Typhi causes disease through a wide repertoire of toxins and virulence effectors that mediate bacterial invasion of human cells, intracellular survival and immune evasion. A major focus of our laboratory is a key virulence determinant called the typhoid toxin that enters human cells where it causes breaks in our DNA, which activates a cellular alarm system known as the DNA damage response.
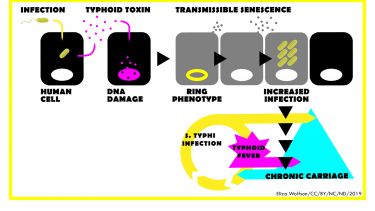
S.Typhi is transmitted via the faecal-oral route (yellow in Figure), a problem which is exacerbated in impoverished countries that lack access to clean water and good sanitation. Following ingestion, S.Typhi infects cells of the intestine before establishing a systemic, often fatal, infection underlying typhoid fever (pink in Figure).
Our research also investigates how S.Typhi and its typhoid toxin cause chronic infections in humans - ‘chronic carriage’ (turquoise in Figure). S.Typhi establishes chronic carriage by evading immune responses and causing an asymptomatic infection (yellow arrow), or following typhoid fever in 5% of cases (pink arrow). Chronic carriers transmit the pathogen in the population and are difficult to diagnose due to the lack of obvious clinical symptoms. The most infamous ‘chronic carrier’ was a household cook called Typhoid Mary who caused numerous outbreaks of typhoid between 1900-1906. Chronic carriage is a stealth strategy of S.Typhi that continues today and helps perpetuate the infection cycle (turquoise arrow), which impedes global efforts to eradicate typhoid. By studying decisive disease mechanisms, our research contributes to global efforts combatting multidrug-resistant typhoid with the aim of improving the health and wealth of vulnerable communities in low- and middle-income countries.
Read more about the typhoid problem here:
https://naturemicrobiologycommunity.nature.com/users/295084-daniel-humphreys/posts/53817-typhoid-fever-an-age-old-problemFor more information on typhoid, please visit Take on Typhoid:
https://www.coalitionagainsttyphoid.orgResearch projects on typhoid and chronic infection
Pathogenic bacteria manipulate the host DNA damage response (DDR) to execute virulence strategies and establish infections. This is exemplified by Salmonella Typhi that causes typhoid fever and fuels the infection cycle by chronic bacterial carriage. Upon infection, S.Typhi deploys the typhoid toxin, which is endocytosed by target host cells where the toxin activates the DDR.
How the toxin hijacks the DDR and how this contributes to typhoid and chronic carriage is unclear. Understanding the toxin is especially important as related toxins are encoded by diverse bacterial pathogens that cause disease in humans and food-chain animals worldwide.
DNA damage responses underlying typhoid and chronic Salmonella carriage
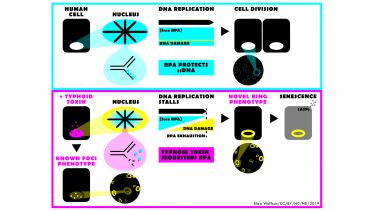
We discovered a novel disease mechanism revealing that the typhoid toxin hijacks the activities of Replication Protein A (RPA), a key component of the DNA replication fork, which elicits DNA damage and cellular senescence.
Normally, RPA coats and protects single-stranded DNA (ssDNA) generated at DNA replication forks, thus acting as a cellular safeguard against DNA damage and permitting cell cycle progression (diagram in turquoise box). Remarkably, we found that the typhoid toxin hijacks this RPA mechanism to induce DNA replication stress and senescence. By inducing ssDNA breaks, toxin nuclease activity sequesters RPA and exhausts the pool of free RPA needed for DNA replication.
This causes irreparable DNA damage observed as signature accumulation of the DNA damage marker γH2AX at the nuclear periphery - Response Induced by a bacterial Genotoxin (RING). We found that the novel RING phenotype marks replication catastrophe and cellular senescence (pink box). Investigating this novel virulence mechanism is providing new information on disease while revealing potential therapeutic targets.
Investigating how pathogens accelerate the ageing of human host cells
We are investigating the significance of pathogen subversion of cellular ageing in typhoid and chronic Salmonella carriage. Understanding how chronic infection develops, and identifying effective diagnostic, treatment and prevention strategies is vital to typhoid elimination efforts.
Infections are often harder to combat and recover from as we age, which is partly due to senescence, and the idea that bacterial pathogens target this phenomenon could be important to infection and contribute to age-related pathologies such as cancer. We find that the typhoid toxin of S.Typhi induces chronic DNA replication stress via the RING phenotype, which results in cellular senescence, thus accelerating cellular ageing (diagram in yellow box).
Interestingly, proteins are secreted from cells with the RING phenotype that induce senescence in neighbouring cells. These senescent cells are rendered more susceptible to intracellular Salmonella infections (see yellow bacteria in grey cells). We are currently investigating the mechanisms of senescence in the host-pathogen interaction.
- Publications
-
Show: Featured publications All publications
Featured publications
Journal articles
- Typhoid toxin hijacks Wnt5a to establish host senescence and Salmonella infection. Cell Reports, 42(10). View this article in WRRO


- Senescence and host–pathogen interactions. Cells, 9(7).


- Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nature Communications, 10(1).


- MYO6 is targeted by Salmonella virulence effectors to trigger PI3-Kinase signalling and pathogen invasion into host cells. Proceedings of the National Academy of Sciences, 114(15), 3915-3920. View this article in WRRO


- Inhibition of WAVE Regulatory Complex Activation by a Bacterial Virulence Effector Counteracts Pathogen Phagocytosis. Cell Reports, 17(3), 697-707.


- Arf6 coordinates actin assembly through the WAVE complex, a mechanism usurped by
Salmonella
to invade host cells. Proceedings of the National Academy of Sciences, 110(42), 16880-16885.


- The Drosophila Arf1 homologue Arf79F is essential for lamellipodium formation. Journal of Cell Science, 125(23), 5630-5635.


- Salmonella Virulence Effector SopE and Host GEF ARNO Cooperate to Recruit and Activate WAVE to Trigger Bacterial Invasion. Cell Host & Microbe, 11(2), 129-139.


- The Salmonella Effector SptP Dephosphorylates Host AAA+ ATPase VCP to Promote Development of its Intracellular Replicative Niche. Cell Host & Microbe, 5(3), 225-233.


All publications
Journal articles
- London calling: The 5th UK Cellular Microbiology Network Meeting. Molecular Microbiology, 120(6), 906-909. View this article in WRRO


- Typhoid toxin hijacks Wnt5a to establish host senescence and Salmonella infection. Cell Reports, 42(10). View this article in WRRO


- Meeting reports. The Biochemist, 45(4), 33-33.


- The great host‐pathogen war:
U.K. Cellular microbiology meeting 2020. Cellular Microbiology, 22(11).

- Senescence and host–pathogen interactions. Cells, 9(7).


- Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nature Communications, 10(1).


- Arf GTPase interplay with Rho GTPases in regulation of the actin cytoskeleton. Small GTPases, 10(6), 411-418. View this article in WRRO


- MYO6 is targeted by Salmonella virulence effectors to trigger PI3-Kinase signalling and pathogen invasion into host cells. Proceedings of the National Academy of Sciences, 114(15), 3915-3920. View this article in WRRO


- Inhibition of WAVE Regulatory Complex Activation by a Bacterial Virulence Effector Counteracts Pathogen Phagocytosis. Cell Reports, 17(3), 697-707.


- The Arf GTPase-Activating Protein Family Is Exploited by Salmonella enterica Serovar Typhimurium To Invade Nonphagocytic Host Cells. mBio, 6(1).


- Arf6 coordinates actin assembly through the WAVE complex, a mechanism usurped by
Salmonella
to invade host cells. Proceedings of the National Academy of Sciences, 110(42), 16880-16885.


- The Drosophila Arf1 homologue Arf79F is essential for lamellipodium formation. Journal of Cell Science, 125(23), 5630-5635.


- Salmonella Virulence Effector SopE and Host GEF ARNO Cooperate to Recruit and Activate WAVE to Trigger Bacterial Invasion. Cell Host & Microbe, 11(2), 129-139.


- WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proceedings of the National Academy of Sciences, 108(35), 14449-14454.


- Enteropathogenic Escherichia coli Recruits the Cellular Inositol Phosphatase SHIP2 to Regulate Actin-Pedestal Formation. Cell Host & Microbe, 7(1), 13-24.


- Nucleotide Binding by Lhs1p Is Essential for Its Nucleotide Exchange Activity and for Function in Vivo. Journal of Biological Chemistry, 284(46), 31564-31571.


- The Salmonella Effector SptP Dephosphorylates Host AAA+ ATPase VCP to Promote Development of its Intracellular Replicative Niche. Cell Host & Microbe, 5(3), 225-233.


- Salmonella takes control: effector-driven manipulation of the host. Current Opinion in Microbiology, 12(1), 117-124.


- Sequence divergence in type III secretion gene clusters of the Burkholderia cepacia complex. FEMS Microbiology Letters, 235(2), 229-235.


- Transition to the open state of the TolC periplasmic tunnel entrance. Proceedings of the National Academy of Sciences of the United States of America, 99.


- Typhoid toxin of Salmonella Typhi elicits host antimicrobial response during acute typhoid fever. EMBO Molecular Medicine.


Book chapters
- WAVE regulatory complex activation. (pp. 363-379).


Datasets
- Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection.


Preprints
- Typhoid toxin of Salmonella enterica induces ISG15 response mediating host cell survival and bacterial dissemination, Cold Spring Harbor Laboratory.


- Typhoid toxin of Salmonella Typhi elicits host antimicrobial response during acute typhoid fever, Cold Spring Harbor Laboratory.


- Typhoid toxin hijacks Wnt5a to potentiate TGFβ-mediated senescence and Salmonella infections, Cold Spring Harbor Laboratory.


- Typhoid toxin hijacks Wnt5a to establish host senescence and Salmonella infection. Cell Reports, 42(10). View this article in WRRO
- Grants
-
- UKRI Future Leaders Research Fellowship (2019)
- HIC-Vac GCRF Grant (2019)
- Royal Society Research Grant (2017)
- MRC New Investigator Research Grant (2016)
- Pathology Research Fellowship (2014)
- Teaching activities
-
I am undergraduate module co-ordinator for Pathobiology BMS106/BMS109 in which I teach infection and immunity. I also lecture on the MSc for Molecular Medicine and the MSc for Antimicrobial Resistance.
- Opportunities
Enquires for postdoc, fellowship and PhD positions are welcome.
We advertise PhD opportunities (Funded or Self-Funded) on FindAPhD.com
For further information and details of other projects on offer, please see the department PhD Opportunities page.