- Scientists at the University of Sheffield have discovered a mechanism behind how plants can acquire long-lasting resistance against attacks from insects
- The newly discovered mechanism enables plants to develop ‘immune memory’ to protect themselves in the future
- The new study in Nature Plants generates opportunities to develop novel crop protection strategies to reduce reliance on damaging pesticides
A mechanism behind how plants can develop long-term immunity to stress has been discovered by scientists at the University of Sheffield.
Biotic stress experienced by plants can take the form of attacks by insect herbivores or disease-causing pathogens. In crops grown for food production, this stress provides a substantial risk to crop yields and is currently managed with the widespread use of pesticides, which are damaging for the environment and can pose a risk to human health.
Due to the urgent need to find better and more sustainable plant protection methods, Professor Jurriaan Ton, from the University of Sheffield’s Institute for Sustainable Food, and his team, investigated how plants are able to acquire long-lasting immunity against these stressors.
The findings, published in Nature Plants, explain a mechanism of how plants ‘remember’ the stress from a previous attack, and that this long-term memory is encoded in a family of 'junk DNA’ that can prime defence genes for several weeks against further attacks.
Dr Ton, a Professor of Plant Environmental Signalling from the University of Sheffield’s School of Biosciences and senior author of the study said the findings offer new opportunities to control plant immunity for sustainable crop protection, and reduce our reliance on damaging pesticides for food production.
He said: “We rely on plants to feed the planet, but they are essentially at the bottom of the food chain, they cannot move, so they are incredibly vulnerable to attack from all sides, including insect herbivores and disease-causing pathogens. Like animals, however, plants have evolved the ability to acquire immunity after recovery from biotic stress, but they use different mechanisms to do so.”
“The findings of the study are not only a huge leap forward in our understanding of how plants 'remember' the stress from previous attacks, but also uncovers a new epigenetic function of a specific family of 'junk DNA’ (transposons; DNA that does not code for plant proteins). This knowledge could help us to develop new breeding strategies, and select crop varieties for food production that are primed to fight off pests and diseases.”
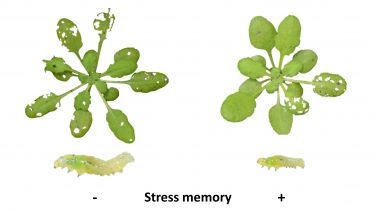
The study investigated the long-term effects of the plant stress hormone jasmonic acid on Arabidopsis thaliana, commonly known as thale cress, a relative to cabbage and mustard. Exposed to caterpillars, the group of seedlings treated with jasmonic acid sustained lower levels of damage than the control group.
Although the short-term effects of jasmonic acid on plant defences are well documented, the long-term effects are not, and the team discovered that immune memory of the stress of being treated with jasmonic acid can linger for several weeks, and be transmitted to newly developed leaves to provide long-lasting resistance against the caterpillars.
The results indicated this acquired immunity is controlled by epigenetic mechanisms, involving small RNA molecules that are generated by the AtREP2 family of transposons, which connect with the small RNA-binding protein, AGO1. The RNA-loaded AGO1 proteins then prime distant defence genes for a faster and stronger response to subsequent stress.
The study provides a first model of long-lasting immune memory in plants, and shows how epigenetic modifications to a specific family of junk DNA can prime plants against further damage by pests.
Dr Samuel Wilkinson, Research Associate from the School of Biosciences and first author of the paper, said: “Being that global food security is one of the biggest challenges we will face in the future, it's imperative that we find new ways to ensure the health and growth of the crops we rely on.
“This research is the first step in being able to complement and enhance the effectiveness and durability of conventional crop breeding strategies, by selecting plants with enhanced immune readiness as an alternative to relying on harmful pesticides.”
The researchers are now collaborating with an international crop breeding company to explore if they can exploit other related epigenetic mechanisms, such as stressors for disease-causing pathogens, and combine them into a novel crop protection strategy for more complex plant genomes.
The researchers are now collaborating with an international crop breeding company to investigate if they can exploit related epigenetic mechanisms to prime crops against devastating diseases.
Samuel added: “The study has opened the way for us to develop a more precise and adjustable method to introduce beneficial epigenetic variation in plant genomes. This would not only be of value to crop protection and breeding, but also represent a valuable research tool to explore the complex mechanisms by which epigenetically altered DNA can prime defence genes within and across plant generations.”