Electron transfer dynamics
We are studying biological electron transfer complexes at the single-molecule level to understand the trade-off between specificity and turnover
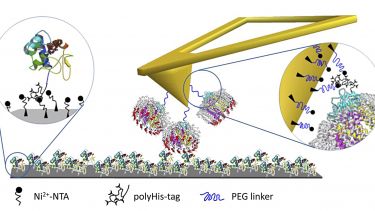
Small diffusible redox proteins play a ubiquitous role in facilitating electron transfer (ET) in respiration and photosynthesis by shuttling electrons between membrane bound complexes in a redox-dependent manner. The association of such small redox carrier proteins with their larger membrane-bound partners must be highly specific, yet also readily reversible in order to sustain rapid ET and turnover on microsecond/millisecond timescales. New developments in force spectroscopy provide the first opportunity to quantify the dynamic forces that sustain these transient interactions and to understand their temporal evolution leading to dissociation. We have adapted a new atomic force microscopy (AFM) technique PeakForce-QNM (PF-QNM), and have used it to directly monitor these intermolecular interactions at the single molecule level, with sub-millisecond time resolution, and with picoNewton force resolution.

Experience Sheffield for yourself
The best way to find out what studying at Sheffield is like is to visit us. You'll get a feel for the atmosphere, the people, the campus and the city.
