Flash photolysis
Information about Sir Harry Kroto's flash photolysis research, during his PhD and beyond.
Harry Kroto's first research result was the Electronic Spectrum of CBr. This was Kroto's PhD research, 1961-1964, at the University of Sheffield.
Harry Kroto speaking about his first result as a researcher:
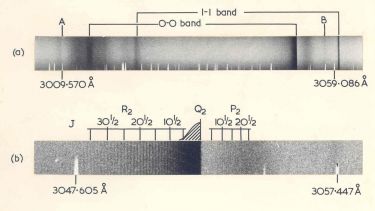
"The year was 1962 when Simons and Yarwod published a paper on the flash photolysis of bromoform CCl3Br at low resolution which indicated they had a nice result, the detection of the CBr radical. My supervisor Richard Dixon suggested that I look for this spectrum at high-resolution using our 21 foot Eagle spectrometer. This was quite easy to do and I did reproduce their results with our instrument.
I called Richard down to the lab to look at the results. He immediately whipped out a magnifier and looked up and said “this is beautiful”. I said “what” and had look myself and saw the most beautiful rotational line structure.
Sir Harry Kroto
"This was my first result as a researcher and of course I shall never forget that moment after a year of negative results. The bands at A and B were a puzzle and I suggested they were upper to lower and lower to upper transitions which Richard in lecture said I had suggested because I did not know they were forbidden! Of course, I did not at the time know anything about forbidden transitions but I was right because rotation mixes the states and they become allowed."
'High-resolution study of the spectrum of the CBr radical' (PDF, 605KB), R Dixon and H Kroto, 1963.
Other papers
- 'Singlet and triplet states of NCN in the flash photolysis of cyanogen azide', H Kroto, Ohio State University, 1966.
Abstract: A new absorption spectrum, at 3327A˚, has been discovered in the flash photolysis of cyanogen azide, NCN3. Rotational analysis of the bands identified them as belonging to a 1Πu←1Δg transition of NCN. The known 3Πu←3Σ−g transition is also observed and its proximity, at 3290A˚, facilitates the study of the relative rates of production and disappearance of the 1Δ−g states. This comparison offers good evidence that the 3Σ−g state is the ground state of NCN and that the main primary process under the conditions used in this study is: NCN3→hνNCN(1Δ)+N2
- 'The 1Πu ← 1Δg electronic spectrum of NCN', H Kroto, T F Morgan and H H Sheena, 1970.
Abstract: The analysis of a new electronic absorption spectrum observed during the flash photolysis of cyanogen azide, NCN3, in the region 3 327 Å indicates that the spectrum belongs to a 1Πu–1Δg transition of NCN.
The 1Δg state is metastable with respect to the 3Σ-g ground state. The bond distance in the 1Δg state is 1.228 5 Å. The value of the Renner splitting parameter, εω2, for the 1Πu state has been determined as −84.2 cm−1.